We are excited to announce the release of MarkLogic’s Data Hub Service for pharma R&D.
This solution demonstrates how pharmaceutical organizations can address critical data challenges that have been hampering R&D processes and the advancement of new products for decades, including limited access to information, inflexible data lakes and difficulties in leveraging machine learning and AI.
The Data Hub Service for pharma R&D (the hub) eliminates these challenges by providing easy access to the widest possible array of R&D data available. Whether it’s articles, chemical compounds, competitive analyses or clinical information—structured and unstructured, public and private—it’s all made available through the hub.
Standing in the Way of Efficient R&D
The main challenge facing IT departments that serve pharma R&D is patchwork infrastructure that creates the data silos that isolate and restrict access to data. Pharmas need to leverage massive data sets, including decades of research and clinical trials information. Without proper access and information management, it’s impossible for drug companies to accelerate results and lower development costs.
Data silos can leave pharmas in the dark, oblivious to potential complications and gaps in R&D. For many research teams, “you don’t know what you don’t know” is a daily and dispiriting refrain. In addition, silos create unnecessary roadblocks as data of varying formats and origin is often difficult, or impossible, to combine for analysis. The inability to ensure that this disconnected information is up to date understandably weakens trust in the data’s integrity.
Collaboration is essential to research, but with a complex, piecemeal data infrastructure, researchers find it difficult to share knowledge—both within and outside of their organizations. And when collaborating to build public knowledge bases such as shared ontologies, they can rarely leverage their shared knowledge to competitive advantage.
But today, with the hub’s innovative approach and agile, next-generation data technology, data challenges no longer need to stand in the way of drug discovery and development.
Advanced Features for Accelerating R&D
The data hub brings powerful features to R&D data, enabling advanced knowledge discovery and collaboration. With up to 10X faster integration of pharma data, your R&D can bring products to market faster and with greater confidence.
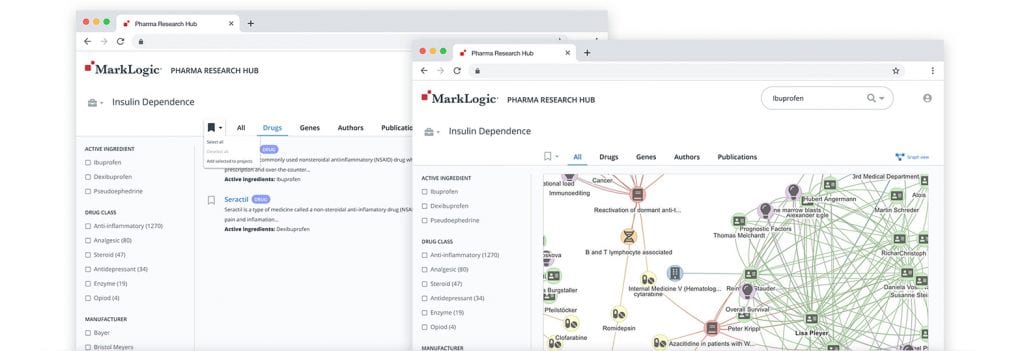
The hub enables researchers to:
- Quickly load any pharma data set Any pharmaceutical data set can be loaded into MarkLogic. Start with publications, authors, drugs, compounds, targets and genes, and then easily expand and scale at any time. Load and harmonize the data that is relevant to your research up to 10 times faster.
- Visualize relationships and discover new ones With MarkLogic’s multi-model database, you can visualize any relationship between data—even discover new relationships. View, navigate and search the graph of connections in your data by leveraging all the inherent relationships. See how researchers are connected to institutions, publications and other peers.
- Fully customize search results Customize your search results by adjusting weighting, relevance scores and exclusions. R&D can perform these customizations without filing tickets with IT, speeding up results and truly leveraging the power of advanced search capabilities.
- Leverage AI and machine learning MarkLogic’s Smart Mastering feature gets you better search results on higher-quality data. You can also apply quality rules to data exports to power AI and machine learning bioinformatics systems outside the hub.
Powered by a Trusted Enterprise Platform
The Data Hub Service for pharma R&D is powered by the MarkLogic database. Our agile NoSQL system provides a multi-model approach to data, built-in search and enterprise-level scalability that allows the hub to be scoped to small data sets or scaled to petabytes. It is reliable, highly available and trusted by thousands of organizations around the world to provide 100% data consistency.
The hub is deployed using the MarkLogic Data Hub Service, an automated cloud service that provides a simple, no-friction deployment option. Pharmas can bring their data to the cloud in minutes with no infrastructure to buy or manage, which frees up your IT team.
Get Started Today
MarkLogic has worked with five of the largest global pharmas to successfully launch research hubs. The hub is a culmination of that success—taking everything we’ve learned about the pharmaceutical industry and combining it with our expertise in running data hubs.
The end result: Our pharma customers can avoid the hassle of building custom-developed IT solutions and focus more of their energy on developing life-saving treatments and the next blockbuster drug.
Learn More
For more information on the Data Hub Service for pharma R&D, please:

Bill Fox
Bill Fox JD, MA is the VP of Vertical Strategy and the Global CSO of Healthcare and Life Sciences at MarkLogic. He is a former attorney and healthcare executive with 25 years of experience and is a nationally recognized thought leader in healthcare predictive analytics, big data, program integrity and data security and privacy.
Bill serves on the HIMSS Health Business Solutions Taskforce and Business Edge Magazine, the thought leaders panel of Predictive Modeling News, and the Board of Directors of the Medical Identity Fraud Alliance.
He is a prior appointee to the Strategic Planning Committee of the National Healthcare Anti-Fraud Association and is a former Senior Fellow at the Jefferson School of Population Health. He has held healthcare leadership positions at Emdeon, Booz Allen Hamilton, and LexisNexis. He is the former Deputy Chief of Economic and Cyber Crime at the Philadelphia District Attorney's Office, Special Assistant United States Attorney for the Eastern District of Pennsylvania and law firm partner. Bill is a graduate of Temple University Graduate School and the Villanova School of Law.